Bonesupport AB is a Scandinavian orthobiologics company that develops and markets Cerament®, an innovative range of radiopaque injectable osteoconductive and drug-eluting bioceramic products that have a proven ability to heal defects by remodeling to host bone in six to twelve months.
Our products are effective in treating patients with fractures and bone voids caused by trauma, infection, disease or related surgery.
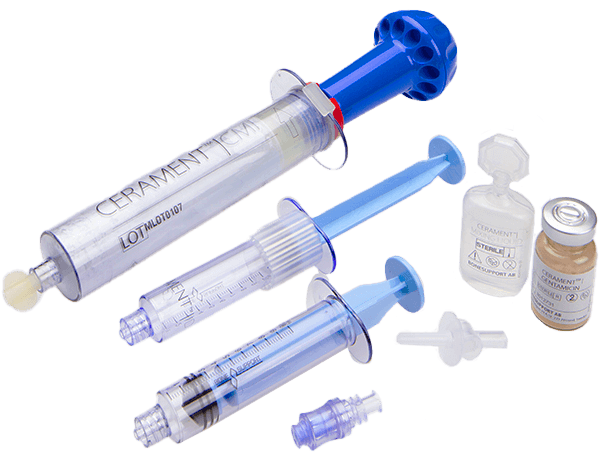
List of Products:
CERAMENT® BVF (Bone Void Filler)
Cerament BVF is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol.
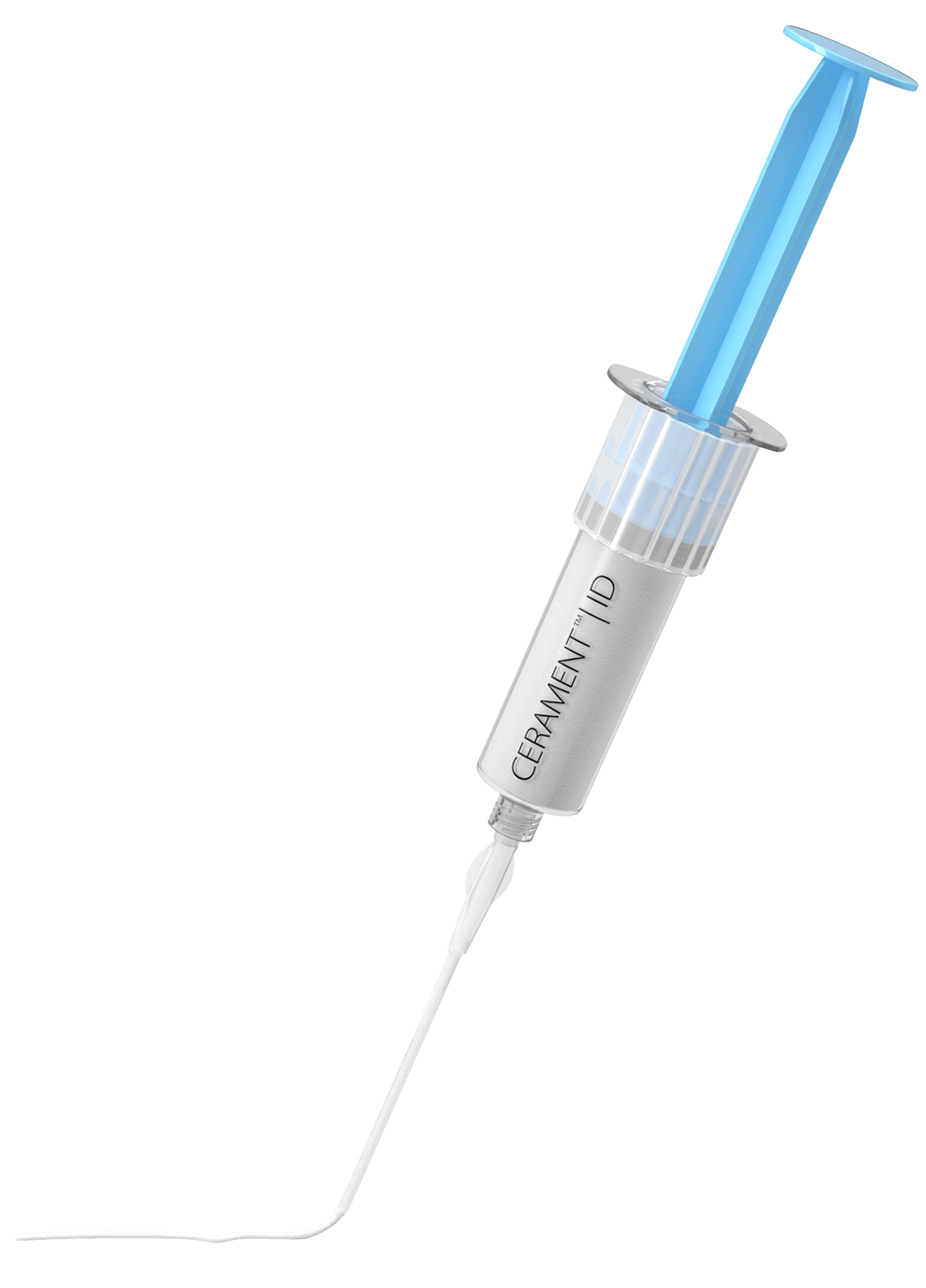
Bone replacement for trauma & benign bone tumours
Cerament is used to fill gaps and voids in bone caused by trauma and benign bone tumours, for example. It is an injectable and mouldable synthetic bone substitute that remodels into the host bone within 6 to 12 months. Cerament is radiopaque, making it ideal for minimally invasive surgery and open surgery. The material is used to expand the hardware during surgery. The unique combination of materials resists crack formation and propagation during drilling.
CERAMENT® G (Gentamicin)
Cerament G is a synthetic remodellable bone filler with high-dose gentamicin for the local treatment of osteomyelitis.
Bone replacement for trauma & benign bone tumours
Cerament is used to fill gaps and voids in bone caused by trauma and benign bone tumours, for example. It is an injectable and mouldable synthetic bone substitute that remodels into the host bone within 6 to 12 months. Cerament is radiopaque,
- CERAMENT | G is the first and only CE-marked gentamicineluting injectable synthetic bone substitute. The product is primarily used for indications with existing infections.Gentamicin is reliable and consistent: The patented Cerament mixing and injection device ensures homogeneous distribution of the antibiotic. The material properties of Cerament itself ensure that the entire antibiotic is made available for elution and delivered in a controlled manner.
- High initial concentration: Maintaining a gentamicin concentration above the MIC values for a clinically relevant period of time (> 28 days) is essential for efficacy. In vitro studies have shown that gentamicin elution from Cerament G has a high initial peak (> 1000 µg / ml) and remains above the MIC for at least 28 days.
- Neutral pH so as not to interfere with antibiotic activity: Cerament G paste is designed for a neutral pH (7.0 – 7.2) so that antibiotic activity is not reduced. Gentamicin efficacy is reduced at pH 6.4 or lower.
- High local gentamicin concentration without high serum gentamicin level: In vivo studies have shown that Cerament G has local gentamicin concentrations 64-150 times higher than the minimum inhibitory concentration (MIC) for gentamicin-sensitive pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa.
CERAMENT® V (Vancomycin)
Cerament V is a synthetic remodellable bone filler with high-dose vancomycin for the local treatment of osteomyelitis.
Bone replacement for trauma & benign bone tumours
Cerament is used to fill gaps and voids in bone caused by trauma and benign bone tumours, for example. It is an injectable and mouldable synthetic bone substitute that remodels into the host bone within 6 to 12 months. Cerament is radiopaque, making it ideal for minimally invasive surgery and open surgery. The material is used to expand the hardware during surgery. The unique combination of materials resists crack formation and propagation during drilling.
Cerament V contains 66 mg vancomycin/ml paste as well as lohexol for enhanced visibility under fluoroscopy. It is the first and only CE-marked vancomycin-eluting synthetic bone substitute on the market. The product is primarily used for indications with present infections.
Vancocmycin is reliable and consistent: regardless of whether Cerament V is injected, shaped or formed into beads, the elution profile of vancomycin is the same. The patented Cerament mixing and injection device ensures homogeneous distribution of the antibiotic. The material properties of Cerament itself ensure that all the antibiotic is made available for elution and delivered in a controlled manner.
- Neutral pH so as not to interfere with antibiotic activity: Cerament V paste is designed for a pH close to neutral (6.6 – 7.0) so that antibiotic activity is not reduced. Vancomycin is effective in the pH range of 6.4 to 8.
- High initial concentration: In vitro studies have shown that vancomycin elution from Cerament V has a high initial peak (> 3000 mg / l) and remains above the MIC for at least 28 days .
- High local vancomycin concentration without high serum vancomycin level: In vitro studies have shown that Cerament V provides a vancomycin level up to 3,000 times the minimum inhibitory concentration (MIC) for methicillin-resistant Staphylococcus aureus (MRSA).

